| |
| Names | |
|---|---|
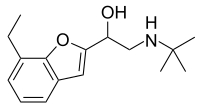
| Preferred IUPAC name
2-(tert-Butylamino)-1-(7-ethyl-1-benzofuran-2-yl)ethan-1-ol | |
| Other names
2-(tert-Butylamino)-1-(7-ethylbenzofuran-2-yl)ethan-1-ol
2-(tert-Butylamino)-1-(7-ethyl-1-benzofuran-2-yl)ethanol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.053.720 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H23NO2 | |
| Molar mass | 261.365 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bufuralol is a potent beta-adrenoceptor antagonist with partial agonist activity.[1] It is metabolized by CYP2D6.[2]
Most beta blockers are aryloxypropanolamine-based. In this rare exception, the benzofuran oxygen is part of a ring instead of derived from the epichlorohydrin precursor.
References
- ^ Pringle, TH; Francis, RJ; East, PB; Shanks, RG (1986). "Pharmacodynamic and pharmacokinetic studies on bufuralol in man". British Journal of Clinical Pharmacology. 22 (5): 527–34. doi:10.1111/j.1365-2125.1986.tb02931.x. PMC 1401192. PMID 2878678.
- ^ Flockhart DA (2007). "Drug Interactions: Cytochrome P450 Drug Interaction Table". Indiana University School of Medicine. Retrieved on July 2011
