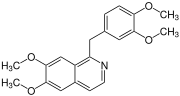
The benzylisoquinoline alkaloids are natural products that can be classified as isoquinoline alkaloidss and are derived from benzylisoquinoline. They also include the benzyl(tetrahydro)isoquinoline alkaloids.
Occurrence
Benzylisoquinoline alkaloids are found in several plant families, including the poppy family (Papaveraceae), the annonaceae family, and the laurel family.[1] Well-known representatives are primarily found in poppy plants, specifically those from which opium is derived, as well as in actaea.[2] For instance, reticuline has been isolated from Annona reticulata.[3]
Known representatives
Over 2500 biologically active derivatives are known from the benzylisoquinoline alkaloids.[4] Based on their structure, the compounds can be divided into numerous subgroups: the aporphines, the phthalideisoquinoline alkaloids, the morphinans, the protoberberine alkaloids, and the pavins.[5]
Among the known individual substances in this group are papaverine. Additional examples of compounds in this group are the benzyltetrahydroisoquinoline alkaloids reticuline and laudanosine.[1]
-
Papaverine.
-
(+)-Reticulin
-
(+)-Laudanosine
Properties
Papaverine has vasodilator and muscle relaxant properties.[3] Laudanosine acts as a tetanic poison.[1]
Biosynthesis
The biosynthesis of benzylisoquinoline alkaloids has been intensively studied. It begins with the amino acid tyrosine, which is converted to dopamine by hydroxylation and decarboxylation and to 4-hydroxyphenylacetaldehyde by oxidative deamination, respectively. These two compounds are converted into dopamine by enzyme Norcoclaurine synthase catalyzed condensation reaction to form the benzylisoquinoline backbone.[6]
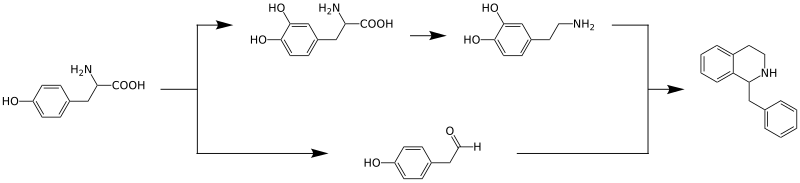
The benzylisoquinoline from this reaction may have different substituents,[6] reticulin is an important intermediate.[5]
References
- ^ a b c Entry on Benzylisoquinoline alkaloids. at: Römpp Online. Georg Thieme Verlag, retrieved {{{Datum}}}.
- ^ Hermann Hager: Hager's Handbook of Pharmaceutical Practice: Volume 2: Methods, 1133 pages, Springer Publishing (1991), ISBN 978-3-540-52459-5, pp. 35 (Benzylisoquinoline alkaloids, p. 35, at Google Books).
- ^ a b Eberhard Breitmaier (1997), Alkaloide, Wiesbaden: Springer Fachmedien, p. 62, ISBN 9783519035428
- ^ Bettina Ruff: Chemical and Biochemical Methods for the Stereoselective Synthesis of Complex Natural Products, 199 pages, Verlag Logos Berlin (2012), ISBN 978-3-8325-3121-8, pp. 8 (Benzylisoquinoline alkaloids, p. 8, at Google Books).
- ^ a b Jennifer M. Finefield, David H. Sherman, Martin Kreitman, Robert M. Williams: Enantiomeric natural products: occurrence and biogenesis. In Applied Chemistry. 124, 2012, p. 4886-4920, doi:10.1002/ange.201107204.
- ^ a b Yang-Chang Wu (2007), "New Research and Development on the Formosan Annonaceous Plants", Studies in Natural Products Chemistry, Studies in Natural Products Chemistry, vol. 33, pp. 957–1023, doi:10.1016/s1572-5995(06)80044-x, ISBN 9780444527172