| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| N2Na2O3 | |
| Molar mass | 121.991 g·mol−1 |
| Appearance | white solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
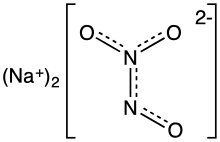
Angeli's salt, sodium trioxodinitrate, is the inorganic compound with the formula Na2[N2O3]. It contains nitrogen in an unusual reduced state. It is a colorless, water-soluble solid, hence a salt. In research, this salt is used as a source of the metastable nitroxyl (HNO), which is a signalling molecule in nature.[1] It is also known by the name sodium trioxodinitrate(II) monohydrate.
Preparation and properties
As first reported by Angelo Angeli in 1896, the salt is prepared by combining hydroxylamine and an organic nitrate, as a source of nitronium (NO+
2):[2][3]
- NH2OH + RONO2 + 2 NaOR′ → ROH + 2 R′OH + Na2N2O3
The structure of the hydrate has been confirmed by X-ray crystallography. The anion is planar. Starting from the ONN end, the bond distances are 1.35 Å (N−O), 1.26 Å (N−N), 1.31 Å (N−O), and 1.32 Å (N−-O). The negative charge is on the oxygen atoms at opposite ends of the molecule. The angles are 112.9° (Osingle−N−N), 118.4° (N−N−Otrans), and 122.5° (N−N−Ocis). This means that the nitrogen–nitrogen bond is a double bond, and that the cis oxygen is slightly repelled by the single oxygen.[4]
Reaction of Angeli's salt with secondary amines in the presence of a proton source results in extrusion of N2 via isodiazenes as proposed intermediates.[5]
References
- ^ Nakagawa, H. (2013). "Controlled release of HNO from chemical donors for biological applications". J. Inorg. Biochem. 118: 187–190. doi:10.1016/j.jinorgbio.2012.10.004. PMID 23140899.
- ^ Angeli, A. (1896). "Sopra la nitroidrossilammina" [On nitrohydroxylamine]. Gazz. Chim. Ital. (in Italian). 26: 17–28.
- ^ Hughes, Martin N.; Cammack, Richard (1999). "Synthesis, chemistry, and Applications of Nitroxyl Ion Releasers Sodium Trioxodinitrate or Angeli's Salt and Piloty's Acid". Nitric Oxide, Part C: Biological and Antioxidant Activities. Methods in Enzymology. Vol. 301. pp. 279–287. doi:10.1016/S0076-6879(99)01092-7. ISBN 9780121822026. PMID 9919577.
- ^ Hope, Hakon; Sequeira, Michael R. (February 1973). "Angeli's salt. Crystal structure of sodium trioxodinitrate(II) monohydrate, Na2N2O3·H2O". Inorganic Chemistry. 12 (2): 286–288. doi:10.1021/ic50120a008.
- ^ Carey, Francis A.; Sundberg, Richard J. (2007). Advanced Organic Chemistry. Part B: Reactions and Synthesis (5th ed.). New York, NY: Springer. ISBN 9781601195494. OCLC 223941000.
