| |
| Names | |
|---|---|
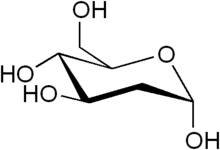
| IUPAC name
2-Deoxy-D-arabino-hexopyranose
| |
| Systematic IUPAC name
(4R,5S,6R)-6-(hydroxymethyl)oxane-2,4,5-triol | |
| Other names
2-Deoxyglucose
2-Deoxy-d-mannose 2-Deoxy-d-arabino-hexose 2-DG | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.295 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H12O5 | |
| Molar mass | 164.16 g/mol |
| Melting point | 142 to 144 °C (288 to 291 °F; 415 to 417 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-Deoxy-d-glucose is a glucose molecule which has the 2-hydroxyl group replaced by hydrogen, so that it cannot undergo further glycolysis. As such; it acts to competitively inhibit the production of glucose-6-phosphate from glucose at the phosphoglucoisomerase level (step 2 of glycolysis).[2] 2-Deoxyglucose labeled with tritium or carbon-14 has been a popular ligand for laboratory research in animal models, where distribution is assessed by tissue-slicing followed by autoradiography, sometimes in tandem with either conventional or electron microscopy.
2-DG is up taken by the glucose transporters of the cell.[3] Therefore, cells with higher glucose uptake, for example tumor cells, have also a higher uptake of 2-DG. Since 2-DG hampers cell growth, its use as a tumor therapeutic has been suggested, and in fact, 2-DG is in clinical trials.[4] It is not completely clear how 2-DG inhibits cell growth. The fact that glycolysis is inhibited by 2-DG, seems not to be sufficient to explain why 2-DG treated cells stop growing.[5] A synergistic effect between 2-DG and various other agents have been reported in the pursuit of anticancer strategies.[6][7][8] Because of its structural similarity to mannose, 2DG has the potential to inhibit N-glycosylation in mammalian cells and other systems, and as such induces ER stress and the Unfolded Protein Response (UPR) pathway.[9][10][11]
YouTube Encyclopedic
-
1/5Views:7 03530 4709962 52020 340
-
IUBMB Life: The wonders of 2-deoxy-d-glucose
-
India Authorizes 2-DG For COVID Management
-
2 Deoxy D-Glucose (2-DG) - Magic bullet- Invention by India for COVID-19 - Tamil
-
Discovery of ACE-2 - main entry receptor for COVID-19 with Professor Anthony J Turner
-
Killing cancer cells by targeting glucose metabolism
Transcription
Use in optical imaging
2-DG has been used as a targeted optical imaging agent for fluorescent in vivo imaging.[12][13] In clinical medical imaging (PET scanning), fluorodeoxyglucose is used, where one of the 2-hydrogens of 2-deoxy-D-glucose is replaced with the positron-emitting isotope fluorine-18, which emits paired gamma rays, allowing distribution of the tracer to be imaged by external gamma camera(s). This is increasingly done in tandem with a CT function which is part of the same PET/CT machine, to allow better localization of small-volume tissue glucose-uptake differences.
Indian adoption for COVID-19 treatment
On May 8, 2021, the Drugs Controller General of India approved an oral formulation of 2-deoxy-D-glucose for emergency use as adjunct therapy in moderate to severe coronavirus patients.[14][15] The drug was developed by the DRDO along with Dr. Reddy's Laboratories, who jointly claimed via a press release, that the drug "helps in faster recovery of hospitalised patients and reduces supplemental oxygen dependence".[15][16][17] The Wire as well as The Hindu noted that the approval was based on poor evidence; no journal publication (or preprint) concerning efficacy and safety are yet available.[16][17]
See also
References
- ^ Merck Index, 11th Edition, 2886.
- ^ Wick, AN; Drury, DR; Nakada, HI; Wolfe, JB (1957). "Localization of the primary metabolic block produced by 2-deoxyglucose" (PDF). J Biol Chem. 224 (2): 963–969. doi:10.1016/S0021-9258(18)64988-9. PMID 13405925.
- ^ Laussel, Clotilde; Léon, Sébastien (December 2020). "Cellular toxicity of the metabolic inhibitor 2-deoxyglucose and associated resistance mechanisms". Biochemical Pharmacology. 182: 114213. doi:10.1016/j.bcp.2020.114213. PMID 32890467.
- ^ Pelicano, H; Martin, DS; Xu, RH; Huang, P (2006). "Glycolysis inhibition for anticancer treatment". Oncogene. 25 (34): 4633–4646. doi:10.1038/sj.onc.1209597. PMID 16892078. S2CID 22155169.
- ^ Ralser, M.; Wamelink, M. M.; Struys, E. A.; Joppich, C.; Krobitsch, S.; Jakobs, C.; Lehrach, H. (2008). "A catabolic block does not sufficiently explain how 2-deoxy-D-glucose inhibits cell growth". Proceedings of the National Academy of Sciences. 105 (46): 17807–17811. Bibcode:2008PNAS..10517807R. doi:10.1073/pnas.0803090105. PMC 2584745. PMID 19004802.
- ^ Cheng, Gang; Zielonka, Jacek; Dranka, Brian P.; McAllister, Donna; Mackinnon, A. Craig; Joseph, Joy; Kalyanaraman, Balaraman (2012-05-15). "Mitochondria-Targeted Drugs Synergize with 2-Deoxyglucose to Trigger Breast Cancer Cell Death". Cancer Research. 72 (10): 2634–2644. doi:10.1158/0008-5472.CAN-11-3928. ISSN 0008-5472. PMC 3700358. PMID 22431711.
- ^ Luo, Zhangyi; Xu, Jieni; Sun, Jingjing; Huang, Haozhe; Zhang, Ziqian; Ma, Weina; Wan, Zhuoya; Liu, Yangwuyue; Pardeshi, Apurva; Li, Song (March 2020). "Co-delivery of 2-Deoxyglucose and a glutamine metabolism inhibitor V9302 via a prodrug micellar formulation for synergistic targeting of metabolism in cancer". Acta Biomaterialia. 105: 239–252. doi:10.1016/j.actbio.2020.01.019. PMC 7105957. PMID 31958597.
- ^ Abebe, Felagot A.; Hopkins, Megan D.; Vodnala, Suraj N.; Sheaff, Robert J.; Lamar, Angus A. (2021-07-20). "Development of a Rapid In Vitro Screening Assay Using Metabolic Inhibitors to Detect Highly Selective Anticancer Agents". ACS Omega. 6 (28): 18333–18343. doi:10.1021/acsomega.1c02203. ISSN 2470-1343. PMC 8296616. PMID 34308064.
- ^ Kurtoglu, M.; Gao, N.; Shang, J.; Maher, J. C.; Lehrman, M. A.; Wangpaichitr, M.; Savaraj, N.; Lane, A. N.; Lampidis, T. J. (2007-11-07). "Under normoxia, 2-deoxy-D-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation". Molecular Cancer Therapeutics. 6 (11): 3049–3058. doi:10.1158/1535-7163.mct-07-0310. ISSN 1535-7163. PMID 18025288. S2CID 6315384.
- ^ Xi, Haibin; Kurtoglu, Metin; Liu, Huaping; Wangpaichitr, Medhi; You, Min; Liu, Xiongfei; Savaraj, Niramol; Lampidis, Theodore J. (2010-07-01). "2-Deoxy-d-glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion". Cancer Chemotherapy and Pharmacology. 67 (4): 899–910. doi:10.1007/s00280-010-1391-0. ISSN 0344-5704. PMC 3093301. PMID 20593179.
- ^ Defenouillère, Quentin; Verraes, Agathe; Laussel, Clotilde; Friedrich, Anne; Schacherer, Joseph; Léon, Sébastien (2019-09-03). "The induction of HAD-like phosphatases by multiple signaling pathways confers resistance to the metabolic inhibitor 2-deoxyglucose" (PDF). Science Signaling. 12 (597): eaaw8000. doi:10.1126/scisignal.aaw8000. ISSN 1945-0877. PMID 31481524. S2CID 201829818.
- ^ Kovar, Joy L.; Volcheck, William; Sevick-Muraca, Eva; Simpson, Melanie A.; Olive, D. Michael (2009). "Characterization and performance of a near-infrared 2-deoxyglucose optical imaging agent for mouse cancer models". Analytical Biochemistry. 384 (2): 254–262. doi:10.1016/j.ab.2008.09.050. PMC 2720560. PMID 18938129.
- ^ Cheng, Z., Levi, J., Xiong, Z., Gheysens, O., Keren, S., Chen, X., and Gambhir, S., Bioconjugate Chemistry, 17(3), (2006), 662-669
- ^ What is 2-deoxy-D-glucose (2-DG) and is it effective against Covid?, The Economic Times, 17 May 2021.
- ^ a b "DCGI approves anti-COVID drug developed by DRDO for emergency use". Press Information Bureau, Government of India. 2021-05-08. Retrieved 2021-05-09.
- ^ a b Borana, Ronak (2021-05-12). "India's Drug Regulator Has Approved DRDO's New COVID Drug on Missing Evidence". The Wire Science. Retrieved 2021-05-18.
- ^ a b Koshy, Jacob (2021-05-11). "Questions remain on DRDO's COVID drug". The Hindu. ISSN 0971-751X. Retrieved 2021-05-18.
